The following edited passage is taken from The Chemistry, Properties and Tests of Precious Stones by John Mastin on the presence of heat-rays and light- rays on different stones.
Another method of isolating certain stones is by the action of heat-rays. Remembering our lessons in physics we recall that just as light-rays may be
5 refracted, absorbed, or reflected, according to the media through which they are caused to pass, so do heat-rays possess similar properties. Therefore, if heat-rays are projected through precious
10 stones, or brought to bear on them in
some other manner than by simple projection, they will be refracted, absorbed, or reflected by the stones in the same manner as if they were light-
15 rays, and just as certain stones allow light to pass through their substance, whilst others are opaque, so do some stones offer no resistance to the passage of heat-rays, but allow them free
20 movement through the substance,
whilst, in other cases, no passage of heat is possible, the stones being as opaque
to heat as to light. Indeed, the properties
of light and heat are in many ways
25 identical, though the test by heat must in all cases give place to that by light, which latter is by far of the greater importance in the judging and isolation of precious stones. It will readily be
30 understood that in the spectrum the
outer or extreme light-rays at each side are more or less bent or diverted, but those nearest the centre are comparatively straight, so that, as before
35 remarked, these central rays are taken
being the standard of light-value. This divergence or refraction is greater in some stones than in others, and to it the diamond, as an example, owes its chief
40 charm. In just such manner do certain stones refract, absorb, or reflect heat; thus amber, gypsum, and the like, are practically opaque to heat-rays, in
contrast with those of the nature of
45 fluorspar, rock-salt, &c., which are receptive. Heat passes through these as easily as does light through a diamond, such stones being classed as diathermal (to heat through). So that all diathermal
50 stones are easily permeable by radiant
heat, which passes through them exactly as does light through transparent bodies.
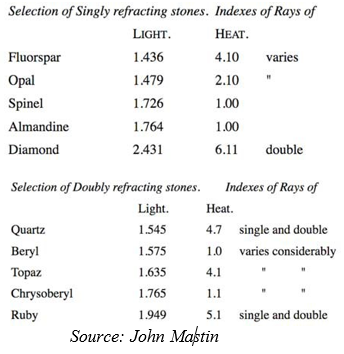
Others, again, are both single and double refracting to heat-rays, and it is
55 interesting to note the heat-penetrating value as compared with the refractive indexes of the stone. In the following table will be found the refractive indexes of a selection of single and
60 double refractive stones, the figures for “Light” being taken from a standard list. The second column shows the refractive power of heat, applied to the actual stones, and consisting of a fine pencil
65 blowpipe-flame, one line (the one twelfth part of an inch) in length in each case. This list must be taken as approximate, since in many instances
the test has been made on one stone
70 only, without possibility of obtaining an average; and as stones vary considerably, the figures may be raised or lowered slightly, or perhaps even changed in class, because in some
75 stones the least stain or impurity may
cause the heat effects to be altered greatly in their character, and even to become singly or doubly refracting, opaque or transparent, to heat-rays,
80 according to the nature of the impurity or to some slight change in the crystalline structure, and so on.
In some of the specimens the gypsum showed a heat-penetration index of
85 0.001, and amber of 0.056, but mostly not within the third point. In all cases
the heat-penetration and refraction were shown by electric recorders. These figures are the average of those obtained
90 from tests made in some cases on several stones of the same kind, and also
on isolated specimens. Not only does the power of the stone to conduct heat vary in different stones of the same kind
95 or variety, as already explained, but there is seen a remarkable difference in value, according to the spot on which the heat is applied, so that on one stone there is often seen a conductivity
100 varying between 0.15 to 4.70.
This is owing to the differences of expansion due to the temporary disturbance of its crystalline structure, brought about by the applied heat. This
105 will be evident when heat is applied on the axes of the crystal, on their faces, angles, lines of symmetry, etc., each one of which gives different results, not only as to value in conductivity, but a result
110 which varies in a curious degree, out of all proportion to the heat applied. In many cases a slight diminution in applied heat gives a greater conductivity, whilst in others a slight
115 rise in the temperature of the heat destroys its conductivity altogether, and renders the stone quite opaque to heat- rays.
This anomaly is due entirely to the
120 alteration of crystalline structure, which, in the one case, is so changed by the diminution in heat as to cause the crystals to be so placed that they become diathermal, or transparent to
125 heat-rays; whilst, in the other instance, the crystals which so arrange themselves as to be diathermal are, by a slightly increased temperature, somewhat displaced, and reflect, or otherwise
130 oppose the direct passage of heat-rays, which, at the lower temperature, obtained free passage.